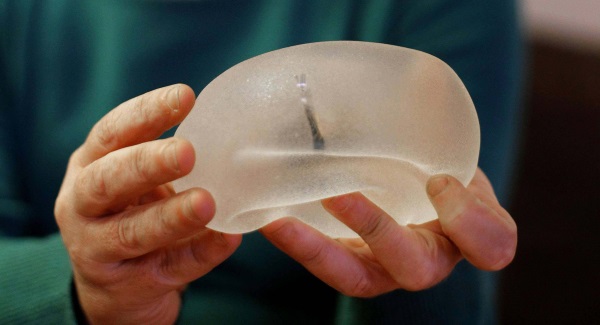
Concerns raised over Silimed breast implants
The Australian Therapeutic Goods Administration (TGA) is urgently investigating what action, if any, is required in Australia after German health officials found surfaces in the Silimed manufacturing plant were contaminated with unknown particles.
But the MHRA statement said that “for the moment there has been no indication that these issues would pose a threat to the implanted person’s safety”.
It also raises concerns about the regulation of the multi-million-pound cosmetic surgery scandal which is still reeling from another breast implant scare.
The company recently said that it relies on Silimed, a third-party manufacturer based in Brazil, as its sole source to manufacture and supply its silicone gel breast implants, tissue expanders, and other products.
“EU health regulators have initiated testing of samples of products to establish if there are any health risks”, it added.
Silimed is responding to the backlash, saying that it is planning to send European health authorities a note showing that its products meet national and worldwide standards, the company told Reuters in an email.
According to MHRA, its investigation with other European regulators is in progress and it recommends that no device of the company should be implanted until further recommendation.
Then on September 24, 2015, the Company notified the fact that the You can introduce.K.’s Medicines and Healthcare products and solutions Regulatory Agency has terminated transmission of its Silimed products and solutions. It (Other OTC: ITGL – news) exports its devices to more than 75 countries worldwide.
The suspension comes five years after French authorities disclosed that one of the top breast implant makers in the world, Poly Implant Prothèse, was not using medical-grade silicone in its implants, earning its president, Jean-Claude Mas, a four-year jail sentence in December 2013.
Any patients who are concerned about their implants should get themselves assessed by their surgeon or clinic.
The British Association of Plastic, Reconstructive and Aesthetic Surgeons said it was aware of the Silimed issue and was working closely with British regulators.