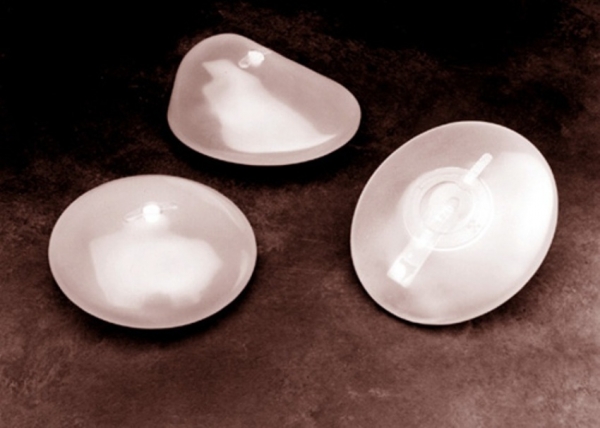
Brazil halts use of Silimed silicone breast implants, follows Europe
Anvisa acted a week after European regulators banned the sale of silicone implants made by Silimed Indústria de Implantes Ltda after a it was found by a German authority that the surfaces were contaminated with particles.
The Brazil-based breast-implant producer has also declared that for the time being it has halted the production and sale of the breast implants that were found to be irregular in the European Union. Thus, the Silimed breast implants could become a danger to patients.
Silimed is complying with the investigation to be sure its products are safe, however it noted in a press release that both the Brazilian and European health agencies have no “technical specifications” on the presence of either quantitative or qualitative levels of particles on devices.
Other countries, such as Australia and Switzerland, have followed suit and called on plastic surgeons to postpone surgeries while health authorities are conducting their own investigation. No patients reported irregularities with the Silimed breast implants.
The company produces Silicon breast, penile and testicular implants which is the largest company in America.
” Need of additional surgery in which may or may not the device is removed”. It’s first in gross sales in Brazil, third on the planet and exports units to greater than seventy five nations worldwide.
This news could be devastating to Silimed, which relies on sales in the United Kingdom to bolster its overall business.
“The vast majority would have been breast implants, and around 60 percent of those would have been for use in cosmetic breast enlargement”, Eurosurgical’s Managing Director Peter Cranstone told Reuters. “We are urgently investigating this issue and are working closely with our European counterparts”.
The MHRA statement said, however, that “for the moment there has been no indication that these issues would pose a threat to the implanted person’s safety”.
Silimed in a prepared statement said that it always had maintained the highest of quality standards and the existence of sterile particles is not representative of any health risk. The Brazilian regulator also stated that all tests are carried out now in order to assess the risks involved.
The Silimed product suspension comes after medical authorities found in 2010 that one of the world’s leading breast implant makers, France’s Poly Implant Prothèse (PIP), was not using medical-grade silicone in its devices, leading them to have double the rupture rate of other implants.