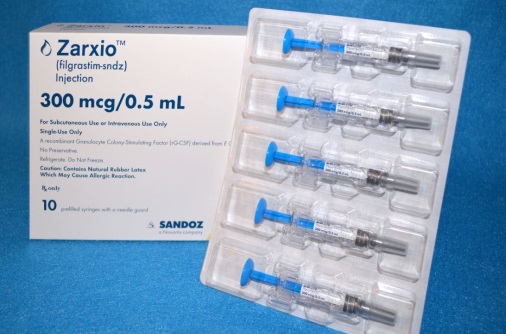
Novartis launches Zarxio in US market
Novartis AG began selling the first copy of a biotechnology drug in the U.S., offering a 15 percent discount to the original after a U.S. appeals court rejected Amgen Inc.’s last-ditch effort to protect its blockbuster cancer drug Neupogen.
The You can introduce.S. Court of Appeals for your Federal Circuit in July said Novartis could grow to business its biosimilar remedy, to come under the brand name Zarxio, after September 4.
The standoff between Amgen and Sandoz (the generics division of Novartis) is part of the process of seeking clarification around the federal regulations which goven biosimilars.
Biosimilars, including a version of Neupogen, have been available in Europe since 2006.
Zarxio bills itself as a biosimilars that can help boost the levels of white blood cells in the body thereby combat cancer and rheumatoid arthritis.
Biosimilars pose a big threat to companies such as Amgen that have over the years relied on biotech drugs.
However, Zarxio’s wholesale list price for a 300 microgram syringe is $275.66, about 15% less than the $324.60 Amgen charges for Neupogen.
As a result, investors have been watching the Zarxio case closely, since it could determine the way in which a wave of other lower-cost biosimilars are received by regulators, courts and healthcare providers. The majority of its sales are generated in the United States.
Amgen (AMGN – Get Report) shares are down 1.5% to $150 in afternoon trading on Thursday after rival Novartis (NVG) launched a biosimilar version of Amgen’s Neupogen treatment.
This decision, which comes as the injunction against Sandoz’s marketing of ZARXIO is set to expire today, clears the way for Sandoz to begin marketing the first biosimilar approved under the BPCIA as early as tomorrow.