Rapid New Ebola Detection Test Could Be Extremely Effective, New Study
Surprisingly, the rapid test also detected some cases that the lab test did not pick up. In the early stages the symptoms – chest pain, cough, nausea – can look like many other illnesses, making it very hard for doctors to triage – to determine who should be quarantined and who to send home.
“A lot of people are frustrated”, says Nira Pollock, an infectious-diseases researcher at the Boston Children’s Hospital in Massachusetts, and senior author of an independent field-validation study of the test kit, published on 26 June in The Lancet.
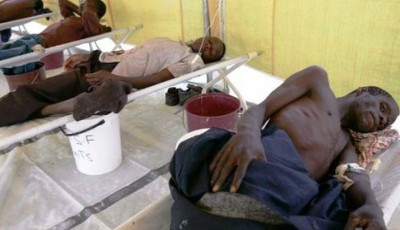
In a major development in the fight against the deadly Ebola virus, a new test has been shown to accurately detect within minutes if an individual is infected by the virus. Existing “gold-standard” methods of diagnosis require blood samples to be drawn and transported to sophisticated central laboratories for processing – which can result in long delays. Conventional lab tests require drawing blood and disposing unsafe needles and syringes.
The evaluation of this test had been conducted in two government centers located in Seirra Leone, one of the most effected nations of the epidemic which has taken the lives of more than 11,200 people in West Africa this week only. The study results confirm that the test can be used in the field and deliver a high level of accuracy, said Robert Garry, professor of microbiology and immunology at the Tulane University School of Medicine.
In February this year, about 106 suspected Ebola patients were admitted to two treatment centres in Sierra Leone and were tested by both RDT (performed on a fingerstick blood sample at the point-of-care) and by standard RT-PCR (performed on plasma in the laboratory). A single drop of blood applied to the strip is all that is needed to produce a diagnostic.
The availability of the ultra-sensitive lab test is however nearly inexistent in Sierra Leone. If the sample is positive for Ebola, a colored line appears on the strip.
The RDT detected all confirmed cases of EVD that were positive by RT-PCR in both point-of-care (28/105 patients) and laboratory testing (45/277 patients), with sensitivity of 100% (identifying all patients with EVD as per the benchmark method), and a specificity of 92% (identifying patients who didn’t have EVD). The study authors noted these cases were only detected using an alternative lab test that is not widely available. Mark Perkins, chief scientific officer of the non-profit organization Foundation for Innovative New Diagnostics (FIND) in Geneva, Switzerland, defends the WHO guidelines noting that rapid tests for antigens report too many false positives.